A Comprehensive Review of Vaccines: Part 2 of 12
When we go to the doctor we usually don’t put too much thought into how our prescription medicine is made or where it comes from. Most of us are familiar with the pharmaceutical companies through their marketing. In fact, you would have to be living under a rock to miss their constant media presence. Even though we know their names, I bet most of us don’t really know too much about them. We simply trust our doctors and have faith that they will prescribe the right drug for the right reasons and that we will be healthier as a result. The truth is that the companies who manufacture and supply the medicine prescribed by our doctors are surprisingly unethical — not only regarding medical and business standards but also legal requirements. In part two we will have a closer look at just who these companies are and how they behave.
Do Pharmaceutical Companies Deserve Our Trust?
When we think of pharmaceutical companies we tend make the false assumption that they are health care companies. In reality, they are businesses, out to maximize shareholder profit, plain and simple. They come right out and say that their goal is not about healing the sick, it is about shareholder profit. This was the justification given by Valeant Pharmaceuticals CEO for raising their drug prices anywhere from 60% to over 500%, putting the costs out of reach for many people.
These companies make products and they want as many people using their products as possible. Ever-increasing growth and profit form the foundation of their business strategy. An auto maker wants as many people as possible to buy their cars and a pizza maker wants to sell as many pies as possible. There is nothing wrong with that concept in theory, it’s what all businesses want. But because these companies are so entrenched within our medical system and are so closely tied to our doctors, we tend to think of them as benevolent organizations out to heal the world. It’s what their marketing slogans say, right? They say they are health care companies and we don’t think to question it. If you think that improving health is priority #1, think again. In business, the bottom line is king.
I want to point out that I believe most of people who work in the pharmaceutical industry are well meaning. I don’t think it would be correct to hold them responsible for the actions of a few decision makers at the top.
Pharmaceutical Legal History
Pharmaceutical companies are some of the most egregious corporate lawbreakers in the world. The offenses include poor manufacturing practices, kickbacks, the sale of tainted drugs and outright deception and fraud. They can afford to employ an army of attorneys and keep legal matters tied up in the court system for a decade or more. They have paid billions of dollars in fines over the last ten years, much of it as punishment for criminal behavior. This may be shocking information, but when we look at the origins of some of these pharmaceutical companies it’s not surprising, after all. You can find out more about the history of Bayer on this website .
The following information on the criminal history of the pharmaceutical industry is taken directly from Propublica.org, as reported by Lena Groeger and taken from Department of Justice data:
*Added by author
Pfizer — September 2009
“Pfizer was fined $2.3 billion, then the largest health care fraud settlement and the largest criminal fine ever imposed in the United States. Pfizer pled guilty to misbranding the painkiller Bextra with “the intent to defraud or mislead”, promoting the drug to treat acute pain at dosages the FDA had previously deemed dangerously high. Bextra was pulled from the market in 2005 due to safety concerns. The government alleged that Pfizer also promoted three other drugs illegally: the antipsychotic Geodon, an antibiotic Zyvox, and the antiepileptic drug Lyrica.”
GlaxoSmithKline — July 2012
“GlaxoSmithKline agreed to pay a fine of $3 billion to resolve civil and criminal liabilities regarding its promotion of drugs, as well as its failure to report safety data. This is the largest health care fraud settlement in the United States to date. The company pled guilty to misbranding the drug Paxil for treating depression in patients under 18, even though the drug had never been approved for that age group. GlaxoSmithKline also pled guilty to failing to disclose safety information about the diabetes drug Avandia to the FDA.”
*China fined GSK $488 million after finding them guilty of creating a “massive bribery network.”
Johnson & Johnson — November 2013
“Johnson & Johnson agreed to pay a $2.2 billion fine to resolve criminal and civil allegations relating to the prescription drugs Risperdal, Invega and Natrecor. The government alleged that J&J promoted these drugs for uses not approved as safe and effective by the FDA, targeted elderly dementia patients in nursing homes, and paid kickbacks to physicians and to the nation’s largest long-term care pharmacy provider, Omnicare Inc. As part of the agreement, Johnson & Johnson admitted that it promoted Risperdal for treatment of psychotic symptoms in non-schizophrenic patients, although the drug was approved only to treat schizophrenia.”
*Here is an excellent expose presented by The Huffington Post and written by Steven Brill about Johnson & Johnson called “Americas Most Admired Lawbreaker.”
Merck —2011, 2012 & 2014
“Merck agreed to pay a fine of $950 million related to the illegal promotion of the painkiller Vioxx, which was withdrawn from the market in 2004 after studies found the drug increased the risk of heart attacks. The company pled guilty to having promoted Vioxx as a treatment for rheumatoid arthritis before it had been approved for that use. The settlement also resolved allegations that Merck made false or misleading statements about the drug’s heart safety to increase sales.”
*Merck continued to promote Vioxx for years after knowing that it was dangerous. For more insight into Merck’s response to the deadly drug Vioxx, you can take a look at this Wall Street Journal article from 2004 or this New York Times article from 2011. Merck created a drug that doubled the incidence of heart attacks and sat on that information for more than four years before they pulled the drug off the market. They subsequently paid $950 million dollars in civil and criminal fines for their handling of the Vioxx catastrophe. We cover this case in more detail in part 4 of this series.
*In 2012, two scientists from Merck, Stephen A. Krahling and Joan A. Wlochowski filed suit against the company claiming the drug maker falsified data on the MMR vaccine. In the lawsuit, Merck is accused of destroying data and submitting falsified data to the FDA. The scientists also accused Merck of threatening to jail them if they went public. Merck has been delaying and trying to get the case thrown out, but in 2015 a judge upheld the action.
Here is just one paragraph from the complaint:
“Third, Merck took steps to cover up the tracks of its fraudulent testing by destroying evidence of the falsified data and then lying to an FDA investigator that questioned Merck about its ongoing testing. Merck also attempted to buy the silence and cooperation of its staff by offering them financial incentives to follow the direction of the Merck personnel overseeing the fraudulent testing process. Merck also threatened a relator in the Qui Tarn Action, Stephen Krahling, a virologist in Merck’s vaccine division from 1999 to 2001, with jail if he reported the fraud to the FDA.”
*In 2014, after being secretly recorded, a senior epidemiologist from the CDC came out and said that he and his co-authors destroyed data from a 2004 study on Merck’s MMR vaccine which linked the drug with an over 300% increase in autism in African American boys under age 3. You can read more about this story, Dr. William Thompson’s admission and CDC’s involvement in the cover-up in part 4 of this series.
Here is a quote from Dr. Thompson:
“It’s all there. This is the lowest point in my career, that I went along with that paper. I have great shame now when I meet families of kids with autism, because I have been part of the problem.”
After seeing this evidence it is not surprising that the then head of CDC, Dr. Julie Gerberding, went on to head up Merck’s multi-billion dollar vaccine division where she has somehow acquired almost 5 million of dollars worth of Merck stock. To be fair, it is unclear to us how Dr. Gerberding acquired so much Merck stock, but it does make the conflict of interest look even worse.
Eli Lilly — January 2009
“Eli Lilly was fined $1.42 billion to resolve a government investigation into the off-label promotion of the antipsychotic Zyprexa. Zyprexa had been approved for the treatment of certain psychotic disorders, but Eli Lilly admitted to promoting the drug in elderly populations to treat dementia. The government also alleged that they targeted primary care physicians to promote Zyprexa for unapproved uses and “trained its sales force to disregard the law.”
AstraZeneca —April 2010
“AstraZeneca was fined $520 million to resolve allegations that it illegally promoted the antipsychotic drug Seroquel. The drug was approved for treating schizophrenia and later for bipolar mania, but the government alleged that AstraZeneca promoted Seroquel for a variety of unapproved uses, such as aggression, sleeplessness, anxiety, and depression. AstraZeneca denied the charges but agreed to pay the fine to end the investigation.”
Abbott — May 2012
“Abbott was fined $1.5 billion in connection to the illegal promotion of the antipsychotic drug Depakote. Abbott admitted to having trained a special sales force to target nursing homes, marketing the drug for the control of aggression and agitation in elderly dementia patients. Depakote had never been approved for that purpose, and Abbott lacked evidence that the drug was safe or effective for those uses. The company also admitted to marketing Depakote to treat schizophrenia, even though no study had found it effective for that purpose.”
Endo — February 2014
“Endo Health Solutions Inc. and its subsidiary Endo Pharmaceuticals Inc. agreed to pay $192.7 million to resolve criminal and civil liability arising from Endo’s marketing of the prescription drug Lidoderm. As part of the agreement, Endo admitted that it intended that Lidoderm be used for unapproved indications and that it promoted Lidoderm to healthcare providers this way.”
Amgen — December 2012
“Amgen agreed to pay a $762 million fine to resolve criminal and civil charges that the company illegally introduced and promoted several drugs including Aranesp, a drug to treat anemia. Amgen pleaded guilty to illegally selling Aranesp to be used at doses that the FDA had explicitly rejected, and for an off-label treatment that had never been FDA-approved.”
Bayer – 1980’s
*The video clip below describes how, in the mid-1980’s, attorneys presented evidence to the US government that Bayer product, Factor VIII, was contaminated with the AIDS virus. Factor VIII is a blood-clotting protein used to prevent hemophiliacs from bleeding to death. Rather than taking their infected product off the market immediately, Bayer stopped selling it in the US and instead, sold it to countries in Europe, Asia and Latin America. The catastrophe was kept quiet, even by the FDA. Bayer’s statement to the press is that they behaved “….responsibly, ethically and humanely…”. Bayer admitted no fault and took no responsibility for infecting untold numbers of people around the world with the (then deadly) AIDS virus.
If you’re wondering how this turned out for Bayer, CBS ran this story in 2011, that explains the fallout for Bayer. After three decades, they paid tens of millions of dollars in compensation for infecting thousands of people abroad with AIDS. It sounds like a gag order was part of the settlement agreements, which explains why we still think of Bayer as the benevolent health care company that makes aspirin.
Obviously, when pharmaceutical corporations break the law, the consequences are a little different than if you or I break the law. Not only are people not serving time, but the U.S. government, via the Medicare and Medicaid programs, continue to do business with each and every one of these companies. The FDA continues to work closely with Bayer to license new products and treatments. and Bayer proudly states that they are “….committed to continually bringing new therapies to hemophilia A patients who need them.” So there you have it. The message is that we need pay no attention to Bayer’s track record. Some people died, but Bayer has officially accepted no responsibility and life goes on. It appears that no regulation, no oversight and no criminal system can or will say otherwise. Maybe, somehow, we can’t expect to uphold the same standards for corporations as for individuals.
If the pharmaceutical industry is not to blame, then where do we look? It would not be in their own best interests to address the safety or effectiveness of any of their products beyond what is required by law and regulation or to spend any money beyond the inescapable costs of doing business. We could take a clue from Nathan Shasho, who wrote the history book Perspective: Making Sense of it All: “Indoctrination and propaganda, coupled with an uninformed public, are powerful tools that have been used throughout history by those who would make us their pawns. Critical thinking, along with a well-informed public, is the only way to combat this indoctrination.
Shasho quotes Albert Einstein: “Unthinking respect for authority is the greatest enemy of truth.” In reality, if I’m pointing one finger of blame at the pharmaceutical industry, I have to be pointing three others back at myself. If I choose to accept their brilliantly simple and oft repeated ‘safe and effective’ slogan and I am content to skim the news for information about vaccines without looking any further, my consent cannot be very informed. In this scenario, I consent by default, out of a misguided trust, a dangerous respect for authority and complacency born out of comfort. Having given our uninformed consent to vaccines for so long puts us in a bad position to object to vaccines as they become increasingly numerous and legally mandatory. The less we know, the less we know.
Exploiting Developing Countries
Pharmaceutical companies have done some pretty awful things in the name of progress, under the heading of science. Maybe even worse things have been done in the service of profit. For example, it is common practice to test vaccines and other drugs on third world children whenever that research cannot be done stateside for ethical reasons or when it is cheaper and faster to go elsewhere. A 2011 PBS segment titled “‘Explosive’ Growth in Foreign Drug Testing Raises Ethical Questions”, reveals that in 2008, more than three fourths of FDA approved drug applications contained data from clinical trials conducted outside the U.S.
In 2014, The Huffington Post published an article titled “Unethical Clinical Trials Still Being Conducted in Developing Countries” that explains how some of the clinical trials carried out in poorer countries would never be allowed in the U.S. for ethical reasons. In a 2005 New York Review of Books review, Dr. Marcia Angell provides a more thorough explanation of the motives and benefits for pharmaceutical companies to conduct clinical trials outside the country. Chief among their motives, she notes, is the option of skirting FDA regulations and oversight.
In 2008, GlaxoSmithKline conducted clinical trials of the Synflorix pediatric pneumonia vaccine on children in Argentina. Fourteen children who participated in the trials died and the Argentinean Federation of Health Professionals accused GSK of misleading and pressuring impoverished, disadvantaged families into enrolling their children in the trials. CNN tells us that GSK was fined a mere $240,000 but that the vaccine has now been approved by regulatory agencies in 85 countries. The vaccine is not approved for use in the U.S., by the way.
There are many examples of drug makers conducting questionable and sometimes, nefarious, research that exploits the less privileged who live in poor or oppressed parts of the world. You can read about pharmaceutical company exploits of the poor in India, their use of unapproved drugs on children in Nigeria, a $250 million fine for bribery in China and unethical clinical trials being conducted around the world. If you want to read about this growing trend in greater detail, you can pick up a copy of Sonia Shah’s 2006 book The Body Hunters: Testings New Drugs on the World’s Poorest Patients, which provides abundant evidence of the unscrupulous, unethical and cruel treatment of people in the developing world by pharmaceutical companies. This behavior doesn’t exactly square with that of an industry concerned with health.
The practice of conducting drug trials overseas has recently skyrocketed in popularity. A 2011 Vanity Fair article by Donald L. Bartlett and James B. Steele revealed that in 1990, just 271 drug trials were being conducted in foreign countries for drugs marketed and sold in the US. By 2008, the number of trials conducted outside the US was 6,485. They note this is an increase of more than 2,000 percent.
Drug companies hire contract research organizations (CROs) to conduct clinical trials in poor areas of the world where regulatory oversight is often deeply lacking. Outsourcing clinical oversight to CROs is a way for pharmaceutical companies to manage risk and minimize liability for negative outcomes and ethical breaches. In 2001, the US Department of Health and Human Services, Office of Inspector General, published The Globalization of Clinical Trials, A Growing Challenge in Protecting Human Subjects in which they recognize the trend toward outsourcing clinical trials to countries that have little experience in conducting this type of research. A main point of the paper is that the “FDA cannot assure the same level of human subject protections in foreign trials as domestic ones.” This statement becomes even more concerning when you realize the ridiculously small number of clinical trial sites the FDA inspects for research occurring within the U.S.: less than 2% in 2008.
Regarding where pharmaceutical companies are conducting clinical drug trials and who they’re experimenting on, Bartlett and Steele tell us:
“(T)housands are taking place in countries with large concentrations of poor, often illiterate people, who in some cases sign consent forms with a thumbprint, or scratch an “X.” Bangladesh has been home to 76 clinical trials. There have been clinical trials in Malawi (61), the Russian Federation (1,513), Romania (876), Thailand (786), Ukraine (589), Kazakhstan (15), Peru (494), Iran (292), Turkey (716), and Uganda (132). Throw a dart at a world map and you are unlikely to hit a spot that has escaped the attention of those who scout out locations for the pharmaceutical industry.”
Dr. Marcia Angell explains the recruitment of clinical research subjects in poor countries:
“This system makes a mockery of the notion of informed consent—the requirement that subjects be given full information about the nature of the research and have the right to refuse to participate, without penalty or consequences for their usual health care. That requirement is enforced in the US and other well-to-do countries, and partly for that reason, drug companies are having a hard time getting enough volunteers for the growing number of clinical trials. Not so in the third world, where authoritarian regimes and corrupt local government officials and health authorities are eager to be paid off by first-world organizations and to have good relations with them. They “encourage” entire villages or prov-inces (sic) to enroll in research programs, while local doctors enrich themselves by providing human subjects.”
Here’s what Bartlett and Steele discovered about FDA oversight of clinical trials conducted outside the US:
“The F.D.A. gets its information on foreign trials almost entirely from the companies themselves. It conducts little or no independent research. The investigators contracted by the pharmaceutical companies to manage clinical trials are left pretty much on their own.”
In 2008 the F.D.A. inspected just 1.9 percent of trial sites inside the United States to ensure that they were complying with basic standards. Outside the country, it inspected even fewer trial sites—seven-tenths of 1 percent. In 2008, the F.D.A. visited only 45 of the 6,485 locations where foreign drug trials were being conducted.
Dr. Angell explains that the FDA may not even know about clinical trials conducted abroad until after the fact, when and if they are presented as evidence for drug approval.
Despite untold costs to human life, the strategy is an effective one. Costs are kept low, regulations are often minimal and the trials get presented more expeditiously to the FDA. New drugs, including vaccines, are approved quickly and stockholders enjoy a profit.
Trading Safety for Profit: Where Our Drugs are Made
Cheap labor and lack of regulation translate into pharmaceutical outsourcing on another front as well. In addition to sending clinical trials overseas, pharmaceutical companies are now outsourcing manufacturing operations. A 2012 USA Today article reports that pharmaceutical imports more than doubled between 2002 and 2012. Public radio talk show host Diane Rehm did a program in February, 2014 titled “The Safety Of Prescription Drugs Made Outside The U.S.”.
Rehm notes that 80 percent of the pharmaceutical drugs we use in the U.S. actually come from India and China.
Rehm cites a Government Accountability Office study saying that “…. the FDA inspects foreign drug manufacturing facilities about once every 11 years. The pace was even slower — once every 14 years — for those in China and India, America’s largest pharmaceutical suppliers.” Our government admits that up to two thirds of the places that make our drugs overseas have never been inspected by the FDA, Rehm tell us. This October, 2015 story in Bloomberg Business News, lists some of the reasons they say the FDA is concerned about drugs manufactured in China. All of this begs a few questions: Does the cost savings for pharmaceutical companies offset the risks of importing the majority of our prescription medications from China and India, beyond the reach of FDA oversight? If the FDA is not inspecting overseas manufacturing facilities, who is?
A possible answer to this question is that perhaps the World Health Organization (WHO) will help to regulate and inspect the booming number of manufacturing facilities that are popping up outside the US. For example, China is expected to become a global leader in vaccine production, having been approved by the World Health Organization (WHO) in 2013 for an encephalitis vaccine and in 2015 for a flu vaccine. That means that the UN can now purchase vaccines from China. The initial WHO nod toward China as a vaccine producer coincided with reports of the deaths of 17 newborns soon after receiving a Chinese-made Hep B vaccine in 2013. The South China Morning Post reported in January, 2014, that the vaccine was not to blame: “The World Health Organisation’s China wing said in a statement last night there was “no evidence that the quality of the vaccine has caused these … events”.
Nevertheless, CBS News reports, three Chinese drug makers suspended operations in the wake of this event because they failed to meet new manufacturing standards, including the maker of the Hep B vaccine these babies received. You can take a look at this article from Mercola.com, which explains the situation in greater detail, including information about how Merck helped to build the Chinese company who made this vaccine, Shenzhen Kangtai. In September, 2015, Radio Free Asia reported on 360 children from a single county in China who experienced adverse reactions after receiving out of date vaccines the previous year. You may not be surprised to learn of the report that “…. the Shenqiu county center for disease control and prevention (CDC) has published the results of a report, which concluded that the diseases weren’t linked to vaccinations.”
Unsafe Manufacturing Practices and Tainted Drugs
Pharmaceutical manufacturers are regularly fined for breeches of the laws, rules and standards for safe manufacturing of vaccines. In 2011, 60 Minutes ran a story on whistleblower Cheryl Eckard, former GlaxoSmithKline (GSK) employee and manager of global quality assurance at a drug manufacturing facility in Puerto Rico. A New York Times article notes that this “was GlaxoSmithKline’s premier manufacturing facility, producing $5.5 billion of product each year.” Eckard reported her dire concerns to GSK, expecting them to shut down operations. Her concerns were regarding the broken nature of the facility, equipment and processes used to manufacture drugs at this facility. She reported problems with bacterial contamination, water contamination, wrong drug strengths and even drugs being mislabeled. For example, diabetes drugs were mixed up with over the counter antacids and it appears that some 25 mg. Paxil was labeled as 10 mg.
Ms. Eckard references the discovery of the Paxil mix-up by a grandmother who discovered her 8 year old grandson’s reaction after taking two and half times his prescribed dosage of Paxil due to the mix up. Eckard was laid off 8 months after her initial report, at which time GSK had taken no action to close the plant. Ms. Eckard, in conjunction with the US government, filed suit against GSK saying that the pharmaceutical company had defrauded the government by selling adulterated drugs to millions via the Medicaid program. GlaxoSmithKline paid $750 million dollars to settle the suit. They take it in stride, though, because at the end of the day, the wheels kept turning and the manufacturing process experienced only a mild hiccup as GSK moved operations and settled up with the feds and the whistleblower.
The New York Times article mentioned above notes that this case is not an exception to the rule and that these types of suits “…. have for years been rising in size and scope, but the collective threat to the industry has been largely unnoticed because the growing mountain is obscured by a wall of judicial secrecy. Each successful claim begets more suits, with more being filed almost every week.”
Maybe the most successful PR campaign is the one that flies under our radar — the one that keeps ugly facts out of the news and out of our awareness. The ingenious campaign creates a reality in which we think of pharmaceutical companies as part of the health care industry, focused on creating and sustaining health. The imagined link between the pharmaceutical industry and the medical industry has permeated our culture to such an extent that the media uses the term ‘health care’ to refer to both. By association, the pharmaceutical industry has influenced our perspective and we think of them as partners in health solutions. They bought themselves a prime spot in our awareness of the health care industry. They have insinuated themselves right into our living rooms as health experts and bastions of credibility, via the evening news. They have interwoven themselves with our public health organizations in a brilliant and seamless way.
Pharmaceutical companies exchange employees with organizations like the CDC and FDA and continue to enjoy extremely friendly relationships with both entities, despite flagrant criminal wrongdoing. The pharmaceutical industry influenced Congress to make it legal for them to fund the FDA by paying ‘user fees’ and to pass laws that mean that they own exactly 0% liability for vaccine safety. They have helped to finance the educations and careers of many of those in charge of public health. It’s a stunningly expensive, complex and effective strategy. Is it fair to demonize them for just being so darn good at what they do? If you needed to market a product or service, wouldn’t you want to hire someone this savvy?
Not unlike many other giants of industry, big names that form the financial backbone of our country, pharmaceutical companies have done some unethical and immoral things. In the final analysis, though, this corporate behavior has been very, very good for their bottom line. Those who aspire to corporate greatness might say that when a company is so successful that it can incur a $3 billion fine and never skip a beat, they must be doing something right for their stockholders! Truly masters of commerce, they control the message about their product while creating exorbitant demand. Pharmaceutical companies are a model for how to thrive in business in a capitalist economy.
Why Is Pharma Paying So Much To Our Doctors?
Doctors are the gatekeepers between pharmaceutical products and the consumer and a lot of money flows between the two. Doctors wield the power to put pharmaceutical products in the hands of patients so it is of great benefit to drug makers to influence these doctors to act on their behalf. Doctors have the trust of their patients and there is just no substitute for that when it comes to patient choice and consent. Without prescriptions, there is no revenue and without doctors, there are no prescriptions.
Drug companies identify doctors who hold influence in niche medical markets as targets for strategic partnership. Those who hold sway with other doctors, who are highly regarded within their field, can be particularly helpful pharmaceutical industry partners. By influencing one esteemed physician, the drug companies can influence countless others with whom that doctor holds sway. They’re killing several birds with one very well-placed stone. This NPR story from 2010 explains the psychology behind drug company choices about which doctors to target and how they win their favor.
Then there are other doctors who do research for pharmaceutical companies and the doctors who serve on their advisory boards. Many of these same doctors also serve on the advisory boards of our federal regulatory and oversight agencies. You would not need to acquire the staunch loyalty of too many doctors in key positions and markets in order for your company to be in a position to influence the health care agenda, including federal legislation.
Vaccine proponents tell us to pay no mind to this conflict of interest and that this money does not influence doctors decisions. We know better. It is implausible that doctors, or anyone else, would not be influenced by the amount of money being exchanged. Human nature tells us different. And billions of dollars spent year after year buys a lot of influence. You can take a look at the numbers for yourself in this article from Modern Healthcare in 2015. Then there is this finding from research published in 2014: “Using data from twelve drug companies, more than 330,000 physicians and nearly one billion prescriptions, we find that when a drug company pays a doctor he is more likely to prescribe that company’s drug.” You can read this ABC story for a perspective on how pharmaceutical companies have traditionally bought influence with doctors.
How many doctors are writing favorable reviews of pharmaceutical products while also owning company stock? How many are voting to approve new drugs despite their allegiance to the companies that makes them? How many doctors who serve in decision-making capacities have blatant conflicts of interest?
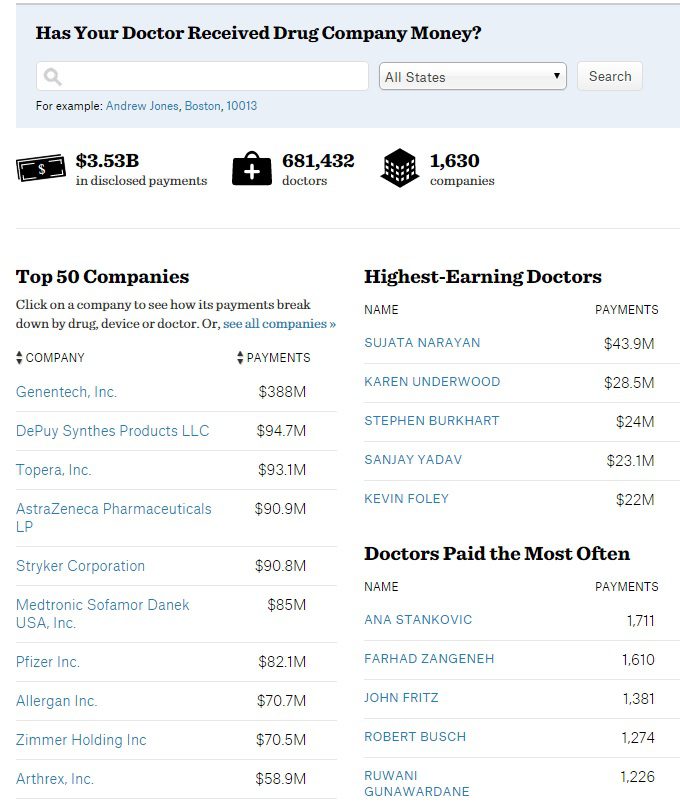
The Affordable Care Act contains within it the Sunshine Act, which went into effect in 2013. It requires pharmaceutical companies to report all cash payments, gifts, and other “transfers of value” it makes to doctors. It is thanks to this law that we now have the numbers reflected in this partial screenshot from a website called Propublica.org. This site tracks payments made to doctors from pharmaceutical companies. This is the amount of money pharma companies paid doctors from August 2013 to December 2014 and excludes research and ownership interests. As you can see in the chart below, doctors received over $3.5 billion in just a 16 month period. That works out to over $200 million every month.
What Industry Whistleblowers are Telling Us
Dr. Peter Wilmshurst — Whistleblower
Dr. Peter Wilmshurst is a semiretired cardiologist from the UK. He has risked his career, his reputation and even personal bankruptcy in favor of telling the truth and publishing his research findings regardless of whether they support the drug company sponsors’ agenda. He was recently recognized by BMJ with the Editor’s Award for courage and persistence in speaking truth to power.
Dr. Wilmshurst explains the problem we face:
“Doctors, institutions and journals that have responsibilities to patients and scientific integrity collude with industry for financial gain.”
In the above video, he offers two examples he experienced firsthand. Early in his career, Dr. Wilmshurst declined a bribe from a drug company: he was offered twice his annual salary in exchange for not publishing a study that revealed horrific side effects of a new heart medication. Decades later, Dr. Wilmshurst was a principle researcher on the steering committee for a clinical trial testing a new implant to treat migraines. In the middle of the study, he learned that several of the other principles had failed to disclose significant conflicts of interest/ties to the pharmaceutical industry. The paper that resulted from this research was fraught with errors and omissions and authored by those with significant conflicts of interest, including a Vice President of the company that manufactured the device. Dr. Wilmshurst and one other researcher declined to be authors on the paper. As it turns out, the paper was published under the names of fifteen authors, many of whom had little if anything to do with the study. None of them were on the design committee and one of the ‘authors’, in fact, died six months before the trial started.
NMT, the Boston-based pharmaceutical company that made the device that was being researched, sued Dr. Wilmshurst for libel for speaking out about the problems with the research when he spoke at a US cardiology conference in 2007. They threatened him with a libel suit for subsequent comments he made on a BBC radio show. (NMT filed for bankruptcy in 2011, effectively ending what Wilmshurst referred to as an attempt to silence free speech.) Wilmshurst describes a world where profit is valued over health and safety by the pharmaceutical industry and where doctors and researchers are either paid by the pharmaceutical companies or are stockholders. It seems clear that the means by which we get information about vaccine safety and efficacy has been utterly corrupted.
Dr. Peter Rost, Former Vice President of Marketing, Pfizer
Dr. Rost is a former anesthesiologist and pharmaceutical VP turned whistleblower. According to Dr. Rost, pharmaceutical companies influence every aspect of the healthcare industry. Here’s what he has to say about the current state of affairs:
“Universities, health organizations, everybody that I’ve encountered in my former career as a pharmaceutical executive, are out there with their hands out. You know everybody’s begging for money, nobody has any money. The government doesn’t have any money. The universities don’t have money. Nobody has money. The only ones that have money are these big multinational corporations, and they have lots of money. And they use that money to basically buy influence. And the way it’s done is – number one, you give these organizations and institutions grants, grants for various kinds of research. You develop research together with them. You establish friends. You make sure that they become beholden to you. And you also pay individual professors and doctors – researchers – directly.”
Blair Hamrick, Former GlaxoSmithKline Rep, Whistleblower
Blair Hamrick is one of two whistleblowers responsible for the historic $3 billion fine against GlaxoSmithKline. He is interviewed by Mike Adams of the Natural News Network. Hamrick describes an industry that pays its sales reps six figure salaries and hefty bonuses for working a few hours a day. He describes the drug company practice of selling drugs ‘off label’, whereby the reps were encouraged to market drugs to doctors, encouraging them to prescribe them for unapproved uses and populations. He tells us how sales reps were coached to minimize and marginalize physician concerns about dangerous drug outcomes. He notes that GSK paid $3 billion in fines in the related fraud settlement out of their cash reserves. You can can read more about this case in this New York Times article from 2012.
Brandy Vaughan, Former Pharma Sales Rep, Whistleblower
Brandy Vaughan used to work for Merck selling Vioxx. She has since come out publicly against the drug maker and the pharmaceutical industry as a whole, after being spurred to conduct her own research before vaccinating her son. She started a non-profit organization called Council for Vaccine Safety, and became an outspoken opponent of California SB277 and similar legislation that removes parental choice and informed consent for vaccines. In the video, Ms. Vaughan describes her experience of several covert attempts to intimidate her and to perhaps silence her, since becoming vocal in her opposition to vaccines and to SB277.
Summary
Trust: reliance on and confidence in the truth, worth, reliability, etc, of a person or thing
Question: If you go to a health food restaurant and notice some unsanitary culinary procedures, and then your neighbor gets food poisoning after eating at the same place, would you continue to frequent that restaurant? Now what if you found out that the restaurant had criminal charges against them and they were caught hiding information regarding the safety of their food? Would it matter that the health department gave the restaurant high scores on their inspection if you saw friend after friend fall ill after eating there? Trust should be earned. How has the pharmaceutical earned our trust?
Pharmaceutical companies are not health care companies. They are businesses which have profit as their first priority. When profit is goal #1, you sometimes have to make some tough calls and, while the stock value goes up, the fallout can be very ugly as it pertains to human life. There can be only one first priority and all other matters take a backseat, including public health.
Are we really that surprised that people do not trust pharmaceutical companies? It’s common practice for pharmaceutical companies to take advantage of the poor, oppressed and illiterate in developing nations in order to expedite bringing drugs to market. They have outsourced vaccine production all over the world because of cheap labor, less regulation and virtually no FDA oversight. Can we trust the manufacturing standards of other countries? There are still many people who will never buy dog food from China and yet, pharmaceutical companies are outsourcing clinical trials and drug manufacturing operations to them and to developing countries around the world. They would have us consent to injecting our babies with toxins, chemicals and viruses that come from places even the FDA does not go.
How do we reconcile the fact that a major pharmaceutical company turned its back on blatantly unsafe manufacturing practices and knowingly disseminated tainted drugs for a period of years? Far from being the outlaws of the pharmaceutical industry, what if GSK is the rule, not the exception? We have plenty of evidence to suggest this is so. Despite paying huge fines, they’re thriving. Essentially, they paid this huge sum out of’ ‘petty cash’ and they’ve moved on to brighter shores. Now they’re hard at work making that flu shot your doctor recommended.
The long list above, of criminal drug company offenses, is by no means a complete collection of all of their wrongdoing. These are just some of the more recent ones. Our doctors are chummier with the drug makers than they are with us, their patients. What engenders more favor than being trusted with someone’s life? Well, the answer is money. And the drug industry plies our doctors with plenty of it.
Those who shape public policy often have ties to the pharmaceutical industry, being on their payroll in many cases. Drug makers aren’t buying influence only with your family doctor. They’re close with the doctors who research their drugs and those who vote to approve them. They are very close to the doctors who run the agencies charged with regulating the industry. Sometimes, in fact, they turn out to be the same people at different times!
Can’t we expect better? It’s tough to imagine a more corrupt system existing in a free society.
This is Part 2 in the 12 part series: A Comprehensive Review of Vaccines
Part 1: Why Are So many People Choosing Not To Vaccinate?
Part 2: A Closer Look at Pharmaceutical Companies
Part 3: Vaccine Policymakers and Conflicts of Interest
Part 4: How The CDC, FDA and WHO Lost the Trust of Vaccine Skeptics
Part 5: Are Vaccines Safe & Effective?
Part 6: What Are The Real Risks of Not Vaccinating in the U.S.?
Part 7: Herd Immunity, Shedding and the Questionable Science Behind Vaccines
Part 8: Did Vaccines Really Eradicate Infectious Diseases in the 20th Century?
Part 9: The Conclusive Evidence Linking Vaccines and Autism
Part 10: Why Don’t We Hear The Whole Story About Vaccines?
Part 11: What Are Independent Experts Telling Us About Vaccines?
Part 12: Vaccines: The Greater Good or Greater Greed? – Conclusion
Speed the Shift is an independent group of researchers on a mission to find the truth about controversial topics. We utilize alternative information sources that are often ignored or outright censored by the corporate media in an effort to gain a broader awareness of relevant issues.